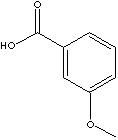
| m-ANISIC ACID | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 586-38-9 |
|
| EINECS NO. | 209-574-9 | |
| FORMULA | CH3OC6H4COOH | |
| MOL WT. | 152.15 | |
| H.S. CODE |
2918.90 |
|
| TOXICITY |
|
|
| SYNONYMS | m-Anisyl acid; m-Methoxybenzoic acid; 3-Anisic acid; | |
| m-Anissäure; ácido m-anísico; Acide m-anisique; | ||
| SMILES | ||
|
CLASSIFICATION |
||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
White to off-white crystalline powder |
|
| MELTING POINT |
102 - 104 C |
|
| BOILING POINT | 170 - 172 C at 10 mmHg | |
| SPECIFIC GRAVITY |
|
|
| SOLUBILITY IN WATER | ||
| AUTOIGNITION | ||
| NFPA RATINGS | Health: 1; Flammability: 0; Reactivity: 0 | |
| FLASH POINT | ||
| STABILITY | Stable under normal temperatures and conditions | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
| Benzoic acid,
the simplest aromatic carboxylic acid containing carboxyl group bonded directly
to benzene ring, is a white, crystalline organic compound; melting at 122 C
(starting sublime at 100 C); boiling at 249 C; slightly soluble in water,
soluble in ethanol, very slightly soluble in benzene and acetone. Its aqua
solution is weakly acidic. It occurs naturally in many plants and resins.
Benzoic acid is also detected in animals. The most of commercial benzoic acid is
produced by the reaction of toluene with oxygen at temperatures around 200 C in
the liquid phase and in the presence of cobalt and manganese salts as catalysts.
It can be prepared also by the oxidation of benzene with concentrated sulphuric
acid or carbon dioxide in the presence of catalysts. Other methods are such as
by the oxidation of benzyl alcohol, benzaldehyde, cinnamic acid; by hydrolysis
of benzonitrile, benzoyl chloride. More than 90% of commercial benzoic acid is
converted directly to phenol and caprolactam. Its use in the production of
glycol benzoates for the application of plasticizer in adhesive formulations is
increasing. It is also used in the manufacture of alkyd resins and drilling mud
additive for crude oil recovery applications. It is used as a rubber
polymerization activators and retardants. Benzoic acid is converted to its salts
and esters for the use of preservative application in foods, drugs and personal
products. Sodium benzoate, sodium salt of benzoic acid, is used preferably as
one of the principal anti-microbial preservatives used in foods and beverages
(but it's concentration is limited usually not exceeding 0.1% because it is
poisonous), as it is about 200 times more soluble than benzoic acid. Sodium
Benzoate is also used in medications, anti-fermentation additives and tabletting
lubricant for pharmaceuticals. The industrial applications are as a corrosion
inhibitor, as an additive to automotive engine antifreeze coolants and in other
waterborne systems, as a nucleating agents for polyolefin, as a dye
intermediate, as a stabilizer in photographic processing and as a catalyst. Wide
range of benzoic esters are used as solvents, dying carrier, disinfectant
additive, penetrating agent and pesticides and manufacturing other compounds.
Benzoic acid derivatives substituted by hydroxy group or ether containing oxygen atom have active bacteriostatic and fragrant properties. They are typically used in pharmaceutical and perfumery industry. The destructive metabolic property of oxygen containing benzoic acid derivatives such as protocatechuic acid (3,4-dihydroxybenzoic acid) and veratric acid (3,4-dimethoxybenzoic acid) is used in the application for pharmaceuticals. Protocatechuic acid is a catabolite of epinephrine. Prazosin, a peripheral vasodilator and antihypertensive, is also an example of the applicaion of veratric acid. Hydroxy and ether substituted benzoic acids feature analogue metabolite of aspirin (acetylsalicylic Acid). They are used as intermediates for pharmaceuticals (especially for antipyetic anlgesic, antirheumatism) and other organic synthesis. They are used as matrix for ionization of peptides, proteins and carbohydrates. Ether is any of a number of organic compounds characterized by an oxygen atom joined with single bonds by two carbon atoms that are part of hydrocarbon groups. The general formula is R-O-R', where R and R' are alkyl or aromatic groups. Ethers are formed by the condensation of two alcohols by heating with sulfuric acid; the reaction is one of dehydration. Ethers can be prepared from alkyl halide reacted with metallic alkoxide (called Williamson synthesis). Ethers are similar to alcohols but are generally less dense, less soluble in water, and have lower boiling points. They are relatively unreactive, which makes them valuable solvents. But ethers will be cleaved at high temperatures by concentrated hydrogen halides. Ethers have relatively low boiling point compare to alkanes as they don't form hydrogen bonds each other. Ethers are more lipophilic than esters [R-C(=O)-O-R']or amides [RCO-NH2]. Ethers are widely used as solvents for various organic reactions because they are relatively the least reactive among common organic compounds except alkanes and fluorocarbons. The common reaction of ethers is cleavage of the C–O bond by strong acids either in linear chain or cyclic structure. Ethers in which oxygen is bonded to primary and secondary alkyl groups can form peroxide compounds in the presence of gaseous oxygen due to two unpaired electrons in oxygen. Ethers can act as Lewis bases in chemical reactions. Commonly, ethers are named simply in listing the alkyl groups in alphabetical order or alkane order such as ethyl methyl ether or methyl ethyl ether, which is methoxyethane in IUPAC nomenclature ( the formula of "alkoxyalkane" ). When ether is a parts of complex molecule or aromatic derivatives, it is described as an alkoxy substituent such as methoxybenzene ( trivial name is anisole). The methoxy prefix indicates the function methyl group joined by single bonds to an oxygen atom, with the general formula -O-CH3. Cyclic ethers have ring structure where the oxygen has become part of the ring. The term of epoxide indicate three membered cyclic ether (also called oxirane) in which an oxygen atom is joined to each of two carbon atoms that are already bonded to each other; four membered cyclic ether is called oxetane; five membered cyclic ether, furan (or oxolane); six membered cyclic ether, pyran (also called oxane) respectively. Their unhindered oxygen atom carries two unshared pairs of electrons - a structure which favors the formation of coordination complexes and the solvation of cations. Cyclic ethers are used as important solvents, as chemical intermediate and as monomer for ring-opening polymerization. Crown Ether is a macrocyclic polyether whose structure contains hydrogen, carbon and oxygen atoms. Each oxygen atoms are confined between two carbon atoms and exhibits a conformation with a hole (accordingly called "crown"). Anisole is one of the simplest aromatic compound to which ether group is linked. But it is different with aromatic compounds like furan where the oxygen is a part of the ring. Anisole, C6H5OCH3 (methyl phenyl ether), is a clear liquid that is soluble in ether and alcohol; insoluble in water; boiling point 155 C. Anisole and its derivatives are used as solvents and in perfumery. Anisole can be obtained from anise seed. Anisic acid, p-methoxybenzoic acid, is a part of cresol class antiseptic compounds. It is also used as an insect repellent and ovicide. Anisole, anisic acid, and their derivatives are also widely used in chemical reaction as intermediates to obtain target materials such as dyes, pharmaceuticals, perfumes, photoinitiators and agrochemicals. |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
White to off-white crystalline powder |
|
| ASSAY |
99.0% min |
|
|
MELTING POINT |
102 106 C |
|
|
LOSS ON DRYIING |
0.5% max |
|
| TRANSPORTATION | ||
| PACKING | ||
| HAZARD CLASS | not regulated | |
| UN NO. | ||
| OTHER INFORMATION | ||
| Hazard Symbols: n/a, Risk Phrases: n/a, Safety Phrases: 24/25 | ||